Clinical research
Find here all our Clinical Research pages.
Inserm and Clinical Research
A very large number of clinical trials are currently in progress in France. Their sponsors – namely, the entities assuming responsibility for them – are most often public research organizations or hospitals (academic research), as well as pharmaceutical companies. Inserm is currently responsible for 250 research projects, 150 of which are sponsored. The vast majority […]
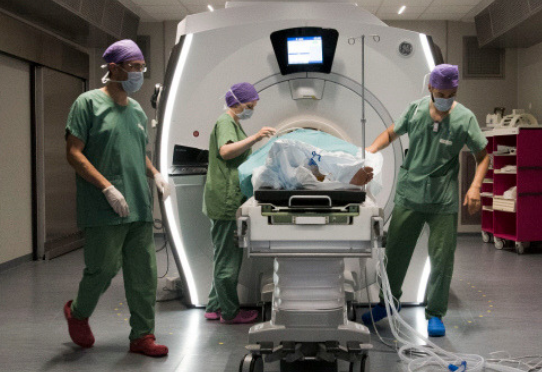
Clinical trials (Interventional studies on a health product)
The objective of a clinical trial on a health product (medicinal product, device, or cell and gene therapy) is to evaluate the safety and efficacy of the latter in healthy volunteers or patient volunteers. The medicinal product will be able to obtain marketing authorization (MA) issued by the French National Agency for Medicines and Health […]
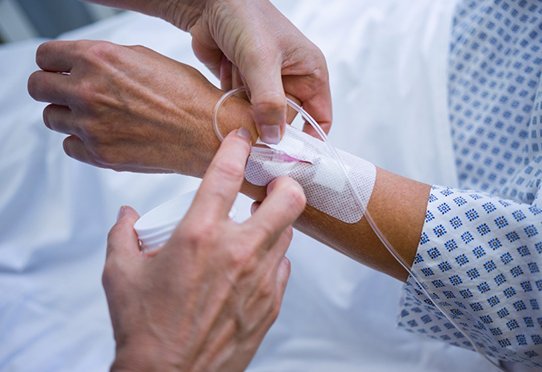
Clinical Research
Clinical research refers to the scientific studies performed on human subjects with a view to furthering biological or medical knowledge. These are prospective studies that require the follow-up of patients or healthy volunteers. This research is essential in order to better understand and treat diseases, and identify potential risk factors. In France, this research is […]

