Our Research
Inserm is involved across the research pathway – from fundamental to clinical, public health, and technology research – in advancing knowledge of living beings and disease. Through these advances and their resulting innovations, Inserm contributes to improving human health.
Find here pages describing the activities carried out in the Institute’s laboratories, as well as the good ethical and deontological practices that are implemented at Inserm.
Scientific Integrity
Scientific integrity is defined as “all of the rules and values that must govern research in order to ensure its honesty and scientific rigor”. As an essential condition for maintaining society’s trust in research stakeholders, scientific integrity is an ongoing consideration at Inserm. Scientific integrity concerns all areas of research: The scientific community as a […]

Value Creation and Transfer of New Discoveries
One of the essentials missions adopted by Inserm involves capitalizing on research carried out by its scientists, by accelerating the transition from the laboratory to the patient’s bedside, and combining an academic approach with industrial strategies. The objective: transforming scientists’ discoveries, inventions and know-how into innovations for society. Increasingly effective and non-invasive diagnostic tests, innovative biological […]

Technology Research
Health technology research covers all technological developments essential to biomedical progress, both on a fundamental level (observing and understanding the mechanisms of living beings) and on a clinical level (transferring knowledge to therapeutic solutions). This concerns five major fields: imaging, technologies associated with drug development, biotechnology and bioengineering, surgery and other interventional techniques, and technologies […]
Fundamental Research
Expanding horizons in life and health sciences, and improving our understanding of biological phenomena, fundamental research is first and foremost a source of knowledge. The results of this exploratory research can be hard to predict, but it can also be particularly effective in revealing the totally novel concepts that are the drivers of progress and […]

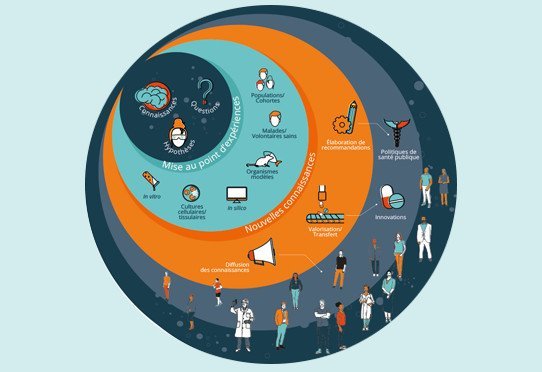
Research Continuum
Knowledge is at the heart of all Inserm research projects: beginning from what is already known, scientists ask questions, construct hypotheses, and develop experiments that will generate new knowledge. From benchtop to bedside, Inserm is involved across the research continuum, so that it can transform these advances into progress in human health. The knowledge generated […]
Use of Animals for Research Purposes
Owing to their scientific validity, scientists rely on animal models to understand diseases and develop treatments. The use of animals for research purposes raises a number of ethical questions and warrants social debate. Inserm uses these models, and is aware of its responsibilities. Animals have been used in research throughout the history of modern medicine. […]

Inserm and Clinical Research
A very large number of clinical trials are currently in progress in France. Their sponsors – namely, the entities assuming responsibility for them – are most often public research organizations or hospitals (academic research), as well as pharmaceutical companies. Inserm is currently responsible for 250 research projects, 150 of which are sponsored. The vast majority […]
Clinical trials (Interventional studies on a health product)
The objective of a clinical trial on a health product (medicinal product, device, or cell and gene therapy) is to evaluate the safety and efficacy of the latter in healthy volunteers or patient volunteers. The medicinal product will be able to obtain marketing authorization (MA) issued by the French National Agency for Medicines and Health […]
Participatory Research
Giving citizens their place alongside researchers in the production of knowledge and innovation is the objective of participatory research. This approach, which concerns all scientific fields and subjects in extremely varied forms, promotes science that is responsive to societal issues. « Citizen science and participatory research are types of scientific knowledge production in which stakeholders from […]

Public Health Research
The objectives of public health research are to understand the influence of factors that determine population health and propose interventions and health policies based on scientific knowledge and evidence. These actions serve to improve health and well-being and reduce health inequalities. At Inserm, over 110 teams are dedicated to public health research. Public health researchers […]

