Endocrine Disruptors: Can We Reduce Our Exposure?
Our exposures to endocrine disruptors – substances capable of interacting with our hormonal system and altering its function – are many, varied, and seem difficult to control. At the Institute for Advanced Biosciences in La Tronche, near Grenoble, an Inserm team is conducting a study to determine whether changing our consumption habits could reduce our exposures. The researchers have chosen to study the case of cosmetic products: soap, shampoo, deodorant, skin creams, toothpaste, etc.

In her student room, Ksenija silently observes the cosmetic products laid out before her. She may not be aware, but they potentially contain endocrine disruptors such as phthalates, phenols, and glycol ethers. Behind this jargon hide compounds that are harmful to the body. They are used as plasticizers or preservatives in certain skincare and beauty products, and are suspected to influence growth, neurodevelopment, and fertility. So can we reduce our exposure to these toxic substances? This is what Inserm researchers at the Institute for Advanced Biosciences in La Tronche near Grenoble want to find out. They are asking young female volunteers like Ksenija to stop using their cosmetic products for five days. In parallel, a large number of urine samples will be collected and analyzed to determine their levels of endocrine disruptors. The latter have a very short lifespan and are eliminated rapidly after being absorbed by the body. The whole challenge of the intervention study is to find out whether our exposure to these chemical compounds can be reduced by changing our consumption habits. And, ultimately, give recommendations to those who wish to protect their health.

Claire Philippat (right), Inserm researcher, and Sarah Lyon-Caen (left), Inserm research engineer, are co-leading the Ireco study (Intervention to reduce exposure to environmental contaminants). This is the first intervention study on endocrine disruptors to be performed in France.
Malorie Graça (left) is recruiting volunteers to participate in the study. The target population is young women. The aim is to modify their exposure before a possible pregnancy, as endocrine disruptors are suspected to interfere with the development of the fetus.


Ksenija has just spent five days without using her usual cosmetic products. The clinical research associates Joane Quentin (left) and Valentine Socquet (middle) pay her a visit. They check that the volunteer has followed the protocol correctly and that she has been able to use the mobile app.
During the intervention phase, the volunteer is given substitute products that do not contain the chemicals being studied, as well as a telephone with an app in which she must enter the time of each use of the products as well as other potential sources of exposure such as food.
A blood sample is taken before and after the intervention phase. Should the levels of endocrine disruptors measured in the urine decrease during the intervention phase, these blood samples will be used to evaluate whether there has also been an effect on health.

Ksenija has collected all her urine samples from before and during the five-day period without cosmetic products. The large number of samples makes it possible to monitor changes in the level of endocrine disruptors in the body over the same day.
The volunteers’ urine samples are stored at ‑80°C at the biological resource center of Grenoble Alpes University Hospital. A total of 6,000 blood and urine samples will be stored before being analyzed.

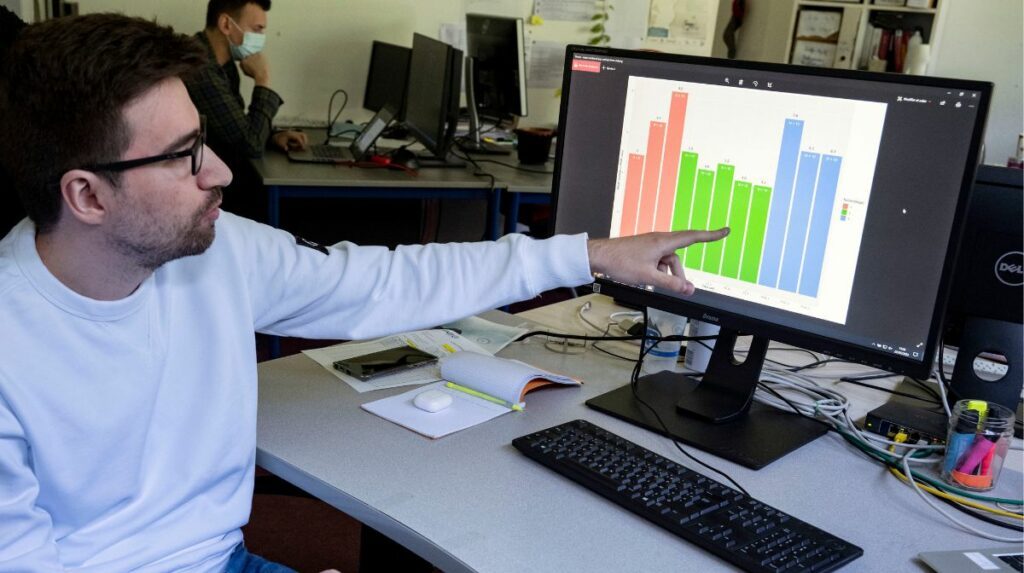
The first data from the pilot study are analyzed by Nicolas Jovanovic, a PhD student in epidemiology at the Institute for Advanced Biosciences.

Abigaël (left) has just completed her participation in the study. The sociologist Maria Belen-Ojeda (right) evaluates her knowledge of endocrine disruptors and her willingness to limit her exposure to these chemicals. This sociological component conducted with a multidisciplinary team is one of the novel aspects of the study.
All in all, one hundred volunteers will be recruited for the Ireco study. The urine samples will be analyzed during the course of 2024 and will reveal whether or not reducing the use of certain cosmetic products reduces exposure to endocrine disruptors.
A report from the Institute for Advanced Biosciences (Inserm unit 1209/Grenoble Alpes University/CNRS), with the Environmental Epidemiology applied to Development and Respiratory Health (EDES) team.
Photos : Inserm/François Guénet
Text : L. A.
