Vision Disorders: Save Our Retinas!
In France, 3 out of every 4 people over 20 years of age suffer from vision disorders. While these can often benefit from optical corrections, some retinal diseases, which are rarer, remain unelucidated. Accredited in 2004, the Clinical Investigation Centre (CIC) and the Quinze-Vingts* Reference Center for Rare Diseases (CMR) have become a reference for the management of macular and neuroretinal genetic diseases.

Expert staff including doctors, orthoptists, nurses and psychologists provide patients, who are at a therapeutic dead-end, with in-depth long-term monitoring of diseases that are often highly incapacitating on a daily basis. « Patients have access to a personalized range of very high resolution examinations making it possible to provide an accurate diagnosis, define biomarkers, and evaluate the efficacy of a treatment, » explains CIC Coordinator, Michel Paques. The data obtained from applied research feed more fundamental questions, and vice versa. Imaging is used to reveal biological phenomena over the long term, which are difficult to observe in animals. Here, a doctor-biologist-physician team is called upon, whose expertise is essential for improving disease knowledge, patient management, and therapeutic innovation: the CIC has established an impressive network of collaboration with the researchers at the Vision Institute**, and this is very clear to see.

Serge Picaud is the Director of the Vision Institute, aimed at better understanding the biology of vision and bringing innovation to the patient’s bedside. Here, « the clinicians are aware of the medical challenges to overcome, and the researchers translate the clinical issues into scientific questions that they are trying to solve, » he explains.

The CIC has a state-of-the-art imaging platform, Paris Eye Imaging, co-directed by Michel Paques and Kate Grieve, where prototypes unique to the world are used to explore different aspects of a disease and provide each patient with a personalized pathway.

These adaptive optical machines, originally used to look at the stars, have been modified to correct eye aberrations and take high-definition images. Kate Grieve, physicist at the CIC, has developed a full field, simple, low cost and high resolution optical coherence tomography (OCT) system that allows the cells to be viewed in 3D. The center has the world’s first two prototypes of this model.

The RTX1 adaptive optics high-definition retinal camera is used to observe retinal cells and vessels over time. It enables the researchers to see the movements of the cells, their appearance and disappearance: they can therefore monitor the course of a disease and control the effect of treatment over time. This camera has already identified new disease processes that are inaccessible to conventional imaging systems, such as certain factors that modulate blood flow during retinal vein occlusions.

Histology, the study of microscopic tissue structures, reveals the parts of the eye that usually cannot be seen under a microscope. To complete the optical measurements and visualize the eye in its globality, tissues can be made completely transparent, thanks to clearing. The HistoParis team is one of three in the world capable of applying this complementary technique in order to obtain viewing angles that are inaccessible during clinical examinations.

The team of Isabelle Audo and Christina Zeitz is one of the first to have applied high-throughput sequencing for the genetic characterization of patients with rare retinal diseases. It is now a very common care practice in this area. The characterization of genetic defects is the basis for gene therapies that replace a faulty gene. These novel therapies improve vision in patients with Leber hereditary optic neuropathy or retinitis pigmentosa, for example.
The stem cell reprogramming protocol also makes it possible to create retinal pigment epithelium grafts to replace these dysfunctional or dying cells in visually impaired patients. Since 2019, four patients with retinitis pigmentosa have benefited from these small patches, transplanted by CIC/CMR surgeon, Stéphane Bertin, and developed by Olivier Goureau in rodents and primates.
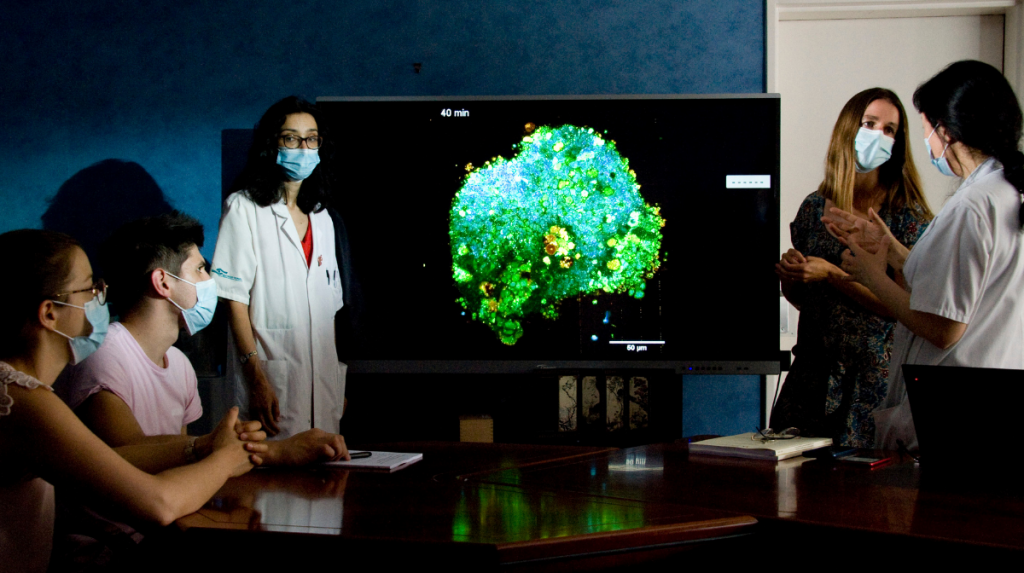
From cells of patients whose genotype and phenotype have been characterized, researchers from Olivier Goureau’s team are able to reprogram pluripotent stem cells and manufacture 3D mini-retinas in culture. These organoids constitute good fundamental research models for understanding the mechanisms that cause blindness in some patients, and may also contribute to finding therapeutic targets or validating different innovative therapies.

The Vision Institute also has a zebrafish platform, with over 1,000 aquariums. Filippo Del Bene’s team applies CRISPR-Cas9 technology to these transparent fish. By genetically modifying the animals to remove or modify key vision genes, it produces models of hereditary visual diseases.

The Vision Institute has four real-world testing platforms for patients: Streetlab, Homelab, a driving simulator, and a virtual reality room. They are used to assess the impact of eye diseases and treatments on patient motor performance and daily life.
In a world first led by José-Alain Sahel, researchers and clinicians partially restored the vision of a blind patient through a new optogenetic therapy, evaluated on the Streetlab platform. This treatment could change the lives of many patients with retinitis pigmentosa, a disease that affects one in every 3,500 people.
Notes:
*CIC 1423 Inserm/Sorbonne Université
** unit 968 Inserm/CNRS/Sorbonne Université
